Gas Laws with CO₂ and H₂ (In Person)
VIDEO LINKS:
Lab Experiments (Write protocols and perform experiments based on these):
Data analysis and calculations (Use these if you need help with your data and interpretations)
The prelab must include Experimental Protocol, Chemical Table and Equipment Table.
The lab report requires all sections (including prelab sections) to be completed in one document.
Experimental Protocol
(Analysis) Watch the experiment videos. Take notes on the protocol. Stop the video and re-watch as necessary to acquire the details of the procedure. Write out the protocol for each part of the experiment. (It can be written in sequential steps. Complete sentences are not necessary.) This is the protocol you will follow, so be detailed.
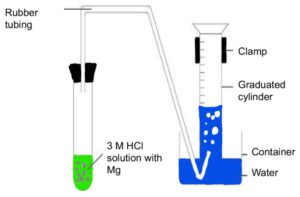

Chemical Table
(Representation) Prepare your chemical table including the materials you will use in the experiment. Here is a general template that you may use.
| Chemical Name | Chemical Formula | Molar Mass (g/mol) | Hazards | reference | PPE |
| Sodium Chloride | NaCl | 58.5 | Skin irritation | https://fscimage.fishersci.com/msds/21105.htm | |
Equipment Table
(Analysis) Identify the equipment (type AND size) needed for the experiment and include the name and an image of each. Be sure to describe the equipment, how to use it, and why it is suitable for this use.
| Equipment Name | Equipment Picture | Intended Purpose |
Data Collection
(Acquiring competencies) Following your detailed protocol based on the videos, perform all the experiments. Record your observations and take pictures of your key steps in the process. Your observations and images need to be incorporated in your data section and this section should be as detailed as possible as you will use this information to complete your discussion.
Data Processing
- (Representation) Write the balanced chemical equation for the reaction observed between the baking soda and vinegar.
- (Interpretation) Using your balanced chemical equation that describes the observed reaction, interpret its meaning on the microscopic (molecules/formula units) and macroscopic scale (moles).
- (Manipulation) Assuming that all the baking soda from your balloon reacted, calculate how many moles of carbon dioxide gas would be formed.
- (Manipulation) Assuming that all the acetic acid (assume 5% by mass with a density of 1.006 g/ml) from your Erlenmeyer flask reacted, calculate how many moles of carbon dioxide gas would be formed.
- (Analysis) Using your calculations, identify the limiting reactant of your reaction and determine the maximum amount (in moles) of carbon dioxide that could be produced.
- (Manipulation) Using your calculated number of moles of carbon dioxide from Question 5, the atmospheric pressure, the vapor pressure of water at your room temperature, and your room temperature value, calculate the expected volume of the balloon.
- (Manipulation) Using your measurement for the circumference of the balloon, calculate the volume of the gas in the balloon.
Radius: r = circumference / (2π)
Volume: V = 4/3 x π x r³
- (Manipulation) Using the expected and the measured volumes of the carbon dioxide, calculate the percent yield of the experiment.
- (Manipulation) Calculate the expected volume for 1 mole of an ideal gas under atmospheric pressure and your room temperature conditions.
- (Manipulation) Using your measured gas volume and calculated moles from Question 5, calculate the molar volume of your collected carbon dioxide gas.
- (Manipulation) Considering the value calculated in Question 9 the correct value for the molar volume under your reaction conditions, calculate the error for the molar volume in your experiment. (Hint: the calculated value from Question 10 is your experimental molar volume.)
- (Representation) Write the balanced chemical equation for the reaction between the magnesium strips and the hydrochloric acid solution.
- (Interpretation) Describe the meaning of the chemical equation on the microscopic (atoms/molecules/formula units) and macroscopic scale (moles).
- (Manipulation) Calculate the moles of hydrogen gas expected from the reaction of HCl with Mg. Use stoichiometry, appropriate units and consider the significant figures.
- (Manipulation) Calculate the actual moles of hydrogen gas produced using the collected volume of gas. (HINT: the gas was collected over water.)
- (Manipulation) Calculate the % yield of the reaction. [Actual moles of H2(g)/expected moles of H2(g)]×100%
- (Assumptions and Analysis) Fill in the following table using the observations and data from your experiments.
| Assumptions made | Testing the assumption | If assumptions are wrong ... |
| All the baking soda is consumed in the reaction. | ||
Discussion
Write a minimum one-page (12 font, single spaced) discussion on the experiment conducted this week. Address at least one question in each category as fully as possible integrating the collected data, providing explanations for the observed trends, and evaluating whether your original assumptions about the experiment were validated by the results. The assignment will be graded on completeness, clarity of the explanations and the meaningful integration of the collected and calculated data. Correct grammar and appropriate format for the chemical formulae and chemical reactions is expected. You may use the outline included at the end of this document on how to build your essay to address each category.
- (Existing knowledge, research, and views) Define ideal gases in your own words and describe the conditions under which a gas will behave as an ideal gas.
- (Analysis) Describe your experimental conditions and compare them to the conditions described in your answer to Question 1. Explain how this knowledge influences the type of equations that you can use to do your calculations.
- (Analysis) Describe and explain the method you used to identify the limiting reactant for your experiment. Use your calculated values to support your arguments.
- (Analysis) Compare your actual yield (volume of the balloon) with your theoretical yield (calculated gas volume) and provide at least one supported argument for the difference between them. Describe the suspected cause and evaluate whether the expected impact is consistent with your calculations. (Hint: consider the solubility of carbon dioxide in water.)
- (Analysis) Compare the % yield values for the baking soda and vinegar experiment with the groups in the class. Comment on the accuracy and precision of the experiment.
- (Analysis) Compare the % yield values for the magnesium and hydrochloric acid experiment with the groups in the class. Comment on the accuracy and precision of the experiment.
- (Analysis) The molar volume should be the same for all gases when they behave as ideal gases. Consider the properties of carbon dioxide (hint: polarity and solubility in water) and use them to explain the differences between the molar volume of an ideal gas and the molar volume of your carbon dioxide.
- (Assumptions and limitations) Identify at least one assumption that you made about CO2 being suitable for this experiment. (Hint: consider polarity and solubility in water.) If this assumption is not valid, describe how it impacts the volume of the gas collected in the experiment.
- (Assumptions and limitations) Identify at least one assumption that you made about H2 being suitable for this experiment. (Hint: consider polarity and solubility in water.) If this assumption is not valid, describe how it impacts the volume of the gas collected in the experiment.
- (Analysis) Propose at least two other gases that would be better suited for such and experiment and two gases that would be worse. Support your choices with relevant information about the proposed gases.